Abstract
Introduction: Decitabine has been recently approved in Europe for treatment of AML patients (pts) aged more than 65 years and unfit to receive standard chemotherapy. However data on its efficacy and tolerability derive mainly from clinical trials performed in selected pts. Herein we report on a population based series of AML pts treated with decitabine and registered in observational prospective studies.
Methods: Between June 2013 and June 2017, 150 elderly AML pts were diagnosed and treated at 17 Hematological center of Northern Italy. Their F/M ratio was 66/84, median age was 75 years (range 65-85); the ECOG performance status (PS) was >2 in 19 pts (13%). Median WBC count was 3.95 x10^3/mL (range 0.34 to 255); marrow blasts were between 20% and 30% in 30 pts (20%). AML was de novo in 79 (53%), therapy-related in 5 (3%) and secondary to myeloid neoplasms (s-AML) in 66 (44%) pts, respectively (52 post-myelodysplastic and 14 post-myeloproliferative disease). Karyotype (K), available in 126 cases, was normal (NK) in 54 pts (43%), t(8;21) in 4 (3%), with intermediate-risk abnormalities in 25 (20%), and with adverse-risk (adv) abnormalities according to ELN risk classification in 42 pts (33%). NPM1 mutation was detected in 9 of 40 (23%) evaluable pts with NK, with FLT3-ITD mutation in 4 of them. Decitabine was administered at the recommended dose of 20mg/mq/daily for 5 days every 4 weeks until toxicity or progression of AML.
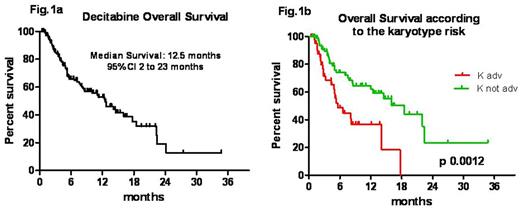
Results: The total number of cycles administered was 761, with a median of 3 cycles/pt (range 1-22). The overall response rate (ORR) was 47% (55/116 evaluable cases). Response was not evaluable in 34 pts (18 too early, 1 early death and 15 deaths during aplasia). A complete remission (CR) was obtained in 34 (29%) cases, partial response (PR) in 10(9%), and hematological improvement (HI) in 11(10%) pts, respectively. In 38 pts (33%) the AML remained stable (SD), whereas 23 (20%) were not responders (NR). The median time to best response was 3.5 months (ms) (range 1-8.5). The median response duration was 5 ms (2-23). Relapse/disease progression occurred in 9/55 responding pts (25.7%). Median survival was 12.5 ms (range 1-35); overall survival (OS) at 1 and 2 years were 52.7% +/- 5 (SE) and 13% +/-7 (SE) (Fig 1a). OS was not influenced by the type of AML (de novo 12.4 ms vs s-AML 16 ms; p 0.8), nor by age, PS, marrow blasts percentage or depth of response (CR vs PR+HI pts; p 0.55). However achieving any response was significantly better than having SD or NR [OS 22.3 ms in responders vs 8 ms in SD (p<0.0001) vs 3 ms in NR, respectively]. Moreover adv risk significantly worsened survival (median survival of 5.5 ms in adv and of 18.6 ms in not-adv K; log- rank p 0.0012) (Fig 1b). Overall, the treatment was well tolerated. Infections were the most frequent adverse event, occurring in 110 cases during 761 cycles (14%) and being responsible for 93% of hospitalizations. They were significantly more common before achieving hematological response during the first 3 cycles, (84 of 356; 23.6%), than in subsequent cycles (26 of 405; 6.4%) [p< 0.0001]. In detail, there were 41 pneumonia (37%), 23 sepsis (21%), 26 FUO (24%), and 20 further type of infections (18%).Ten of 30 (33%) pneumoniae occurring during the first 3 cycles were of fungal origin. Sixty-three pts (42%) died, 40 (63%) of them during the first 3 cycles (40/356 vs 23/405 p 0.0079). The main cause of death was disease progression (67%) followed by infection (19%) in the first 3 cycles and other causes (13%). Only one pt (1%) died of infection while in CR.
Conclusion: These data confirm that decitabine is a feasible and effective first line treatment for elderly AML pts, even in a population-based setting. In this real life experience the ORR was 47% and these results were even better than those reported in clinical trials. In our observational study adverse K remains an unfavourable prognostic factor and pts with not-adverse K and obtaining CR was better survival (median survival: 22.3 ms). Safety profile was acceptable and the most important adverse events were infectious complications with a mortality infections related significantly higher in the first three cycles and in non responder patients.
Rossi: Gilead: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.